Medical device registration in China
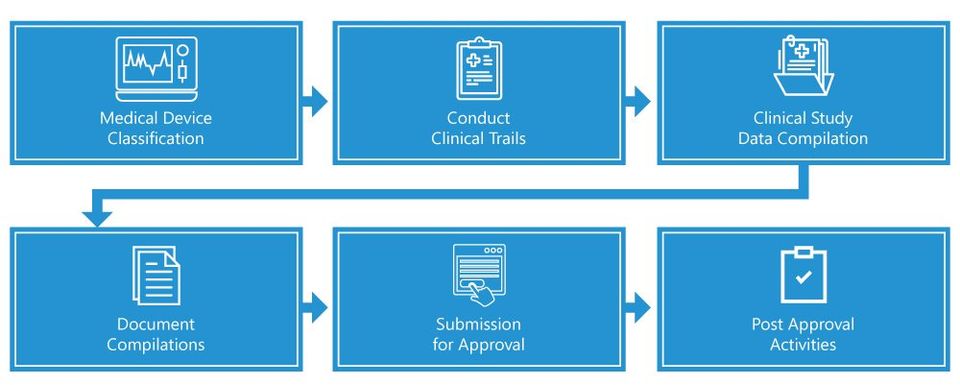
China National Medical Products Administration regulates medical devices and pharmaceutical products across China. The NMPA was also known as China Food and Drug Administration in the past. For medical device manufacturers, understanding these regulations is essential. In this post, we give an overview of the NMPA and current China medical device regulations.
The NMPA classifies medical devices into three categories: Class I, Class II, and Class III. Specifically,
The NMPA classifies medical devices into three categories: Class I, Class II, and Class III. Specifically,
Class I – Medical devices for which routine administration can ensure safety for users and the effectiveness of the device.
Class II – Medical devices that can only be safe and effective with further control in addition to routine administration.
Class III – Medical devices that are implanted into the patient’s body, pose a threat to the patient’s health, or provide sustenance or life support.
Document required to register:
Document required to register:
Class I Device
Product Risk Analysis Document
Product Technical Specification
Product Testing Report (company’s self-testing report or 3rd party report)
Clinical Evaluation Report
Key Manufacturing Information (process, flowchart, material, etc.)
Design/artwork of IFU and product label for the minimum selling unit
Legal Documents
Legal qualification of the foreign manufacturer (i.e. ISO 13485)
Market authorization approval at the country of origin (i.e. CFG+510k or CE)
Authorization letter to the agent in China.
Self-declaration Letters
Letter to declare that the documents submitted meets the CFDA’s regulation for Class I Medical device notification.
Letter to declare that the product conforms to the Class I Medical Device classification catalog.
Letter to declare that the product conforms to the National and/or Industry standards (GB/YY) in China, and the list of these conformed standards.
Letter to declare that all submitted documents are true.
Please note that for all documents sent to the CFDA, the applicant should provide the Chinese translation as well. For the legal documents listed below, the applicant should submit the original or a notified copy.
Class II & Class III Device
A total of 12 document items must be collected and submitted to the CFDA, consisting of 9 legal documents, 2 technical documents, and a testing report issued by a CFDA certified testing center:
Application Form
Legal Documents
Main Safety and Efficacy Specifications List
Summary Data
Overview
Product description
Product model
Description of the package
Intended use and contraindications
Predicated device (if applicable).
Other information
Research Data
Product performance evaluation data
Biocompatibility evaluation data
Biosafety research data
Sterilization and disinfection process validation data
Shelf and package evaluation data
Animal research data
Software validation data
Other data if necessary
Manufacturing information
Manufacturing process description for active/inactive device
Manufacturing site description
Clinical Evaluation Data
Product Risk Analysis Data
Product Technical Specifications
Registration Testing Report
Testing report issued by a CFDA certified lab
Preliminary evaluation comment from the testing lab
Artwork for the IFU and Product Label
Instructions for use (IFU)
Artwork of the product label for the minimum selling unit
Self-declaration Documents
Rely on us for
Before you initiate registration, lets deliberate on
Regulatory Strategy
Device Classification as per NMPA
Assess Clinical Trial Requirements and Exemptions or CER
In case of CER, Evaluation of Technical Clinical Information of a Predictive/Prototype CFDA Approved
Regulatory Strategy
Device Classification as per NMPA
Assess Clinical Trial Requirements and Exemptions or CER
In case of CER, Evaluation of Technical Clinical Information of a Predictive/Prototype CFDA Approved
Review of Existing Clinical Trial Tata Standards
Safety, Quality and Efficacy Assessment
Risk assessment, QMS Audit and Compliance
Self-assurance Statement Preparation
IFU and Draft Label Preparation and Translation
Labeling and Artwork Compliance
Safety, Quality and Efficacy Assessment
Risk assessment, QMS Audit and Compliance
Self-assurance Statement Preparation
IFU and Draft Label Preparation and Translation
Labeling and Artwork Compliance
Fill in the gaps
Local Agent Support, Attend Review Meetings
Liaison with HA until Approval
Import Permit and Custom Support
Post-Approval Regulatory Support and compliance
Liaison with HA until Approval
Import Permit and Custom Support
Post-Approval Regulatory Support and compliance