Medical device registration in Brazil
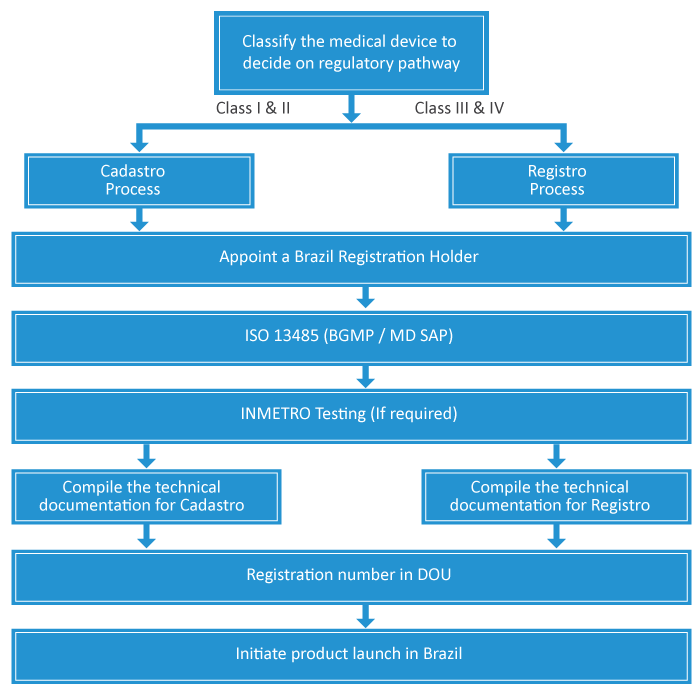
Brazil is one of the biggest countries in South America and Latin America. It spends hugely on healthcare boosting the Medical Devices locally. The country has established regulations in place and it governs Medical Devices through National Health Surveillance Agency (ANVISA - Agência Nacional de Vigilância Sanitária) under Ministry of Health. The registration is done through Cadastro or Registro processes basing on the device classification (Class I, II, III and IV) which also decides the extent of scrutiny involved for approval. Further, all the medical device manufacturers need to comply with Brazilian Good Manufacturing Process (BGMP) which is in lines with ISO 13485. The complex, stringent and extensive Regulatory regime makes the process daunting.
Our Expertise
- Medical Device Classification
- Brazil Local Representation
- Regulatory Support to acquire Brazilian Good Manufacturing Process (BGMP) Certification
- Support for Procuring INMETRO Certification
- Document Compilation for Cadastro and Registro Processes
- Post-market Surveillance