US
One of the first steps toward selling a Class II medical device or IVD in the United States is to file a Premarket Notification with the FDA, also known as an FDA 510(k) submission. Technically, the FDA does not "approve" medical devices or IVDs for sale under the 510(k) process; the agency gives "clearance" for them to be sold in the U.S. We use the term "FDA approval" and "FDA clearance interchangably.
510 k
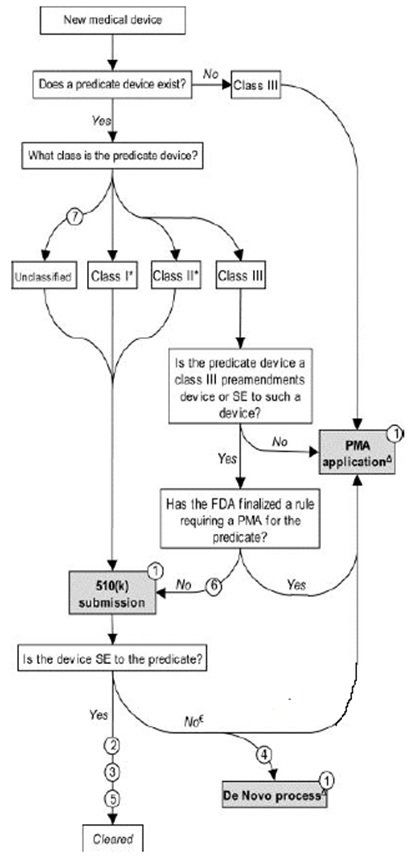
A 510(k) is pre-market notification of medical devices to the US FDA. The number “510” refers to section of the Food, Drug and Cosmetic Act. “k” is the subsection specific to pre-market notification. Details regulating the process, however, are found in 21 CFR 807. approximately 80 percent of all medical devices are covered under the FDA’s 510(k) premarket notification program. Under the 510(k) program, the FDA does not actually “approve” medical devices. Instead, it “clears” them for sale in the market, implying a less rigorous review than that required under the PMA program. However, the 510(k) review program still requires device manufacturers to submit extensive documentation supporting the substantial equivalence(SE) of their devices to other legally marketed medical devices previously cleared by the FDA (referred to as “predicate devices.
Substantial Equivalence:
A 510(k) requires demonstration of substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is at least as safe and effective as the predicate.
A device is substantially equivalent if, in comparison to a predicate it:
• has the same intended use as the predicate; and
• has the same technological characteristics as the predicate;
or
• has the same intended use as the predicate; and
• has different technological characteristics and does not raise different questions of safety and effectiveness; and
• the information submitted to FDA demonstrates that the device is at least as safe and effective as the legally marketed device.
A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics, as applicable.
The mandatory elements of a 510(k) include (amongst others):
1. A sufficiently detailed description of the device, allowing the determination of substantial equivalence.
2. An identification of the predicate device, to which substantial equivalence is claimed.
3. An explanation of the intended use of the device. When these explanations differ from the predicate, an additional explanation must be provided, describing why these differences will not have influence on the safety and effectiveness of the device.
4. If the device has the same technological characteristics as the predicate, a summary must be provided in which the technological characteristics of the new device are compared to the predicate. If the device has different technological characteristics, a summary must be provided in which it is explained in what way the technological characteristics are similar to the predicate’s characteristics.
510 (k) types:
1) Special: For modified devices, processed within 30 days of submission. The Special 510(k) is used for device modifications and utilizes the design controls aspect of the Quality System (QS) regulation (21 CFR 820.30). Special 510(k)s may be submitted for a modification to a device that has been cleared under the 510(k) process. If a new 510(k) is needed for the modification and if the modification does not affect the intended use of the device or alter the fundamental scientific technology of the device, then summary information that results from the design control process can serve as the basis for clearing the application.
2) Abbreviated: Device manufacturers may choose to submit an Abbreviated 510(k) when:
• a guidance documents exists,
• a special control has been established, or
• FDA has recognized a relevant consensus standard.
3) Traditional: The Traditional 510(k) may be used for any original 510(k) or for a modification to a previously cleared device under 510(k).
Substantial Equivalence:
A 510(k) requires demonstration of substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is at least as safe and effective as the predicate.
A device is substantially equivalent if, in comparison to a predicate it:
• has the same intended use as the predicate; and
• has the same technological characteristics as the predicate;
or
• has the same intended use as the predicate; and
• has different technological characteristics and does not raise different questions of safety and effectiveness; and
• the information submitted to FDA demonstrates that the device is at least as safe and effective as the legally marketed device.
A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics, as applicable.
The mandatory elements of a 510(k) include (amongst others):
1. A sufficiently detailed description of the device, allowing the determination of substantial equivalence.
2. An identification of the predicate device, to which substantial equivalence is claimed.
3. An explanation of the intended use of the device. When these explanations differ from the predicate, an additional explanation must be provided, describing why these differences will not have influence on the safety and effectiveness of the device.
4. If the device has the same technological characteristics as the predicate, a summary must be provided in which the technological characteristics of the new device are compared to the predicate. If the device has different technological characteristics, a summary must be provided in which it is explained in what way the technological characteristics are similar to the predicate’s characteristics.
510 (k) types:
1) Special: For modified devices, processed within 30 days of submission. The Special 510(k) is used for device modifications and utilizes the design controls aspect of the Quality System (QS) regulation (21 CFR 820.30). Special 510(k)s may be submitted for a modification to a device that has been cleared under the 510(k) process. If a new 510(k) is needed for the modification and if the modification does not affect the intended use of the device or alter the fundamental scientific technology of the device, then summary information that results from the design control process can serve as the basis for clearing the application.
2) Abbreviated: Device manufacturers may choose to submit an Abbreviated 510(k) when:
• a guidance documents exists,
• a special control has been established, or
• FDA has recognized a relevant consensus standard.
3) Traditional: The Traditional 510(k) may be used for any original 510(k) or for a modification to a previously cleared device under 510(k).
We also support for Premarket Application (PMA) for class 3 devices